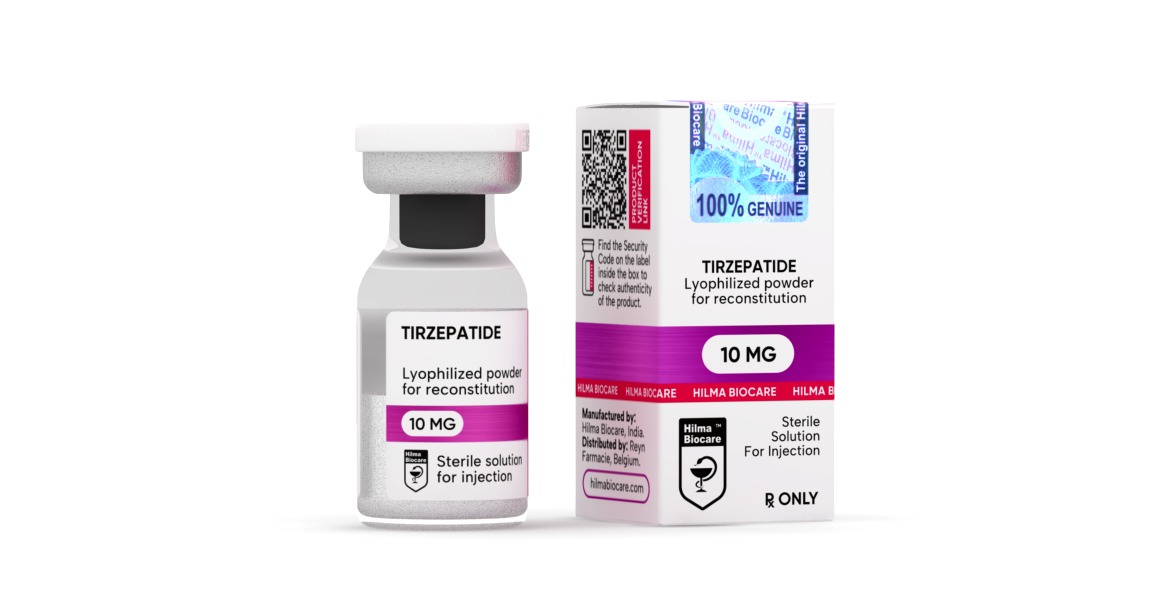
Tirzepatide
InjectableSpecifications Tirzepatide:
Company: Hilma Biocare
Active Half-life : up to 5 days
Group: Body weight correction
Subgroup: Injection / lyophilized powder
Dosage: 10 mg/ml
Application (Men): 2.5–15 mg a week (consult your physician)
Product pack: 1 vial
Content (active): a dual agonist of the GIP and GLP-1 receptors, an analogue of gastric inhibitory polypeptide (GIP) that stimulates insulin release from the pancreas.
Retains water: It depends on the situation, consult your physician
Aromatization: No
Description
Tirzepatide is a dual agonist of the GIP and GLP-1 receptors. Tirzepatide is an analogue of gastric inhibitory polypeptide (GIP), a human hormone that stimulates insulin release from the pancreas. Tirzepatide is a linear polypeptide of 39 amino acids, chemically modified by lipidation to improve its cellular uptake and metabolic stability.
Indication
Treatment of type 2 diabetes mellitus and weight loss. Tirzepatide is used to improve glycemic control in combination with diet and exercise.
Mechanism of Action
Tirzepatide has a higher affinity for GIP receptors than for GLP-1 receptors, and this dual agonist action provides a more pronounced reduction in hyperglycemia compared to a selective GLP-1 receptor agonist. Tirzepatide mimics the action of natural GIP on the GIP receptor. At the GLP-1 receptor, tirzepatide preferentially forms cAMP (a messenger involved in the regulation of glycogen, sugar, and lipid metabolism) rather than attracting beta-arrestin. This combination of its preference for the GIP receptor and the specific signaling properties of GLP-1 increases insulin secretion. Tirzepatide increases levels of both adiponectin and adipokine, which are involved in the regulation of both glucose and lipid metabolism, with a maximum increase of 26% from baseline after 26 weeks at a dose of 10 mg.
Tirzepatide peak concentrations occur 24–48 hours after injection, and steady-state is achieved after 4–5 weeks of treatment. Tirzepatide’s volume of distribution is 5.27 L, and its clearance is 0.0288 L/h. Tirzepatide’s pharmacokinetics are not altered by renal impairment; no dosage adjustments are necessary. No drug interactions have been identified.
Dosage and Administration
Tirzepatide is administered subcutaneously, typically once weekly. The injection site is not important. The standard starting dose for adults is 2.5 mg, which may be increased to 5 mg, 7.5 mg, 10 mg, or 15 mg depending on individual response and tolerability. It is important to follow your doctor’s instructions regarding dosage adjustments.
Side Effects
Common side effects of tirzepatide may include gastrointestinal disturbances, including nausea, upset stomach, constipation, and abdominal pain. Clinical studies have shown that serious side effects are rare and may include hypoglycemia, pancreatitis, kidney and thyroid dysfunction, and allergic reactions. Report any side effects to your doctor immediately if they occur.
Contraindications
Tirzepatide is not recommended for use in patients with a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia syndrome type 2. Tirzepatide’s effect on pancreatitis has not been studied. Tirzepatide is not indicated for patients with type 1 diabetes.
Drug Interactions
Tirzepatide may interact with other medications, including insulin and other antidiabetic medications that may increase the risk of hypoglycemia; medications that affect gastrointestinal motility; and certain blood pressure medications. Before taking Tirzepatide, tell your doctor about all medications and supplements you are taking to avoid potential adverse interactions.
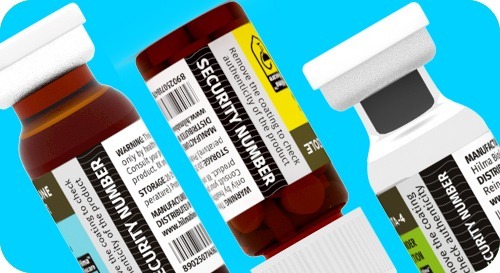
Hilma Biocare™ takes care of consumers and regularly develops various means of protecting its products, which ensures patient safety and trust to verify our products
Want to be Hilma Biocare products Reseller?
Please contact our manager to discuss collaboration details directly via Message form.
Become a Partner